Answer: The correct answer is 1.8 M NaOH.
Step-by-step explanation:
The balanced chemical reaction for the neutralization of NaOH with HCl follows:

To calculate the molarity of the NaOH for neutralization, we use the equation:
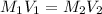
where,
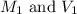 are the molarity and volume of HCl.
are the molarity and volume of HCl.
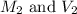 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.
We are given:
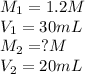
Putting values in above equation, we get:
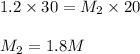
Hence, the correct answer is 1.8 M NaOH.