Answer : The amount of heat required to complete sublime of
 is 20.5 kJ.
is 20.5 kJ.
Explanation :
First we have to calculate the moles of
 .
.
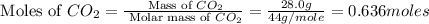
Now we have to calculate the amount of heat required to complete sublime of
 .
.
As, 1 mole of
 on sublimation required heat = 32.3 kJ
on sublimation required heat = 32.3 kJ
So, 0.636 mole of
 on sublimation required heat = 0.636 × 32.3 kJ = 20.5 kJ
on sublimation required heat = 0.636 × 32.3 kJ = 20.5 kJ
Therefore, the amount of heat required to complete sublime of
 is 20.5 kJ.
is 20.5 kJ.