Answer : The volume of hydrogen gas formed at STP are, 8.557 liters
Explanation : Given,
Mass of zinc = 25.0 g
Molar mass of zinc = 65.38 g/mole
First we have to calculate the moles of zinc.
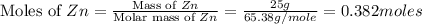
Now we have to calculate the moles of hydrogen gas.
The given balanced chemical reaction is,
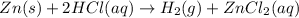
From the balanced chemical reaction, we conclude that
As, 1 mole of Zn react to give 1 mole of hydrogen gas
So, 0.382 mole of Zn react to give 0.382 mole of hydrogen gas
Now we have to calculate the volume of hydrogen gas.
At STP,
As, 1 mole of hydrogen gas contains 22.4 L volume of hydrogen gas
So, 0.382 mole of hydrogen gas contains
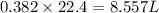 volume of hydrogen gas.
volume of hydrogen gas.
Therefore, the volume of hydrogen gas formed at STP are, 8.557 liters