Answer: The mass of sucrose in the given volume of solution is 1897.92 grams.
Step-by-step explanation:
We are given:
Mass percent of sucrose is 48.5 %. This means that 48.5 grams of sucrose is present in 100 grams of solution.
Density of solution = 1.118 g/mL
To calculate volume of a substance, we use the equation:
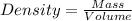
Putting values in above equation, we get:
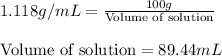
We need to find the mass of sucrose present in 3.50 L or 3500 mL of solution, we apply unitary method:
In 89.44 mL of volume, the mass of sucrose present is 48.5 grams.
So, in 3500 mL of solution, the mass of sucrose present will be
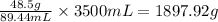
Hence, the mass of sucrose in the given volume of solution is 1897.92 grams.