Answer : The correct option is, It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
Explanation
Dissociation constant of pure water : It is defined as the product of the concentration of hydrogen ion and hydroxide ion.
The equilibrium dissociation reaction for water will be:
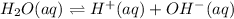
The expression for dissociation constant for water will be:
![k_w=[H^+][OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/yk5cfsy6i1z63jdnf8s8x2tsfuokunbf5m.png)
Experimentally, at
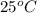 temperature, the dissociation constant of pure water is found to be
temperature, the dissociation constant of pure water is found to be
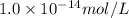 .
.
As we know that pure water is a neutral solution because the concentration of hydrogen ion is equal to the concentration of hydroxide ions.
That means,
![k_w=[H^+][OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/yk5cfsy6i1z63jdnf8s8x2tsfuokunbf5m.png)
![k_w=[H^+][H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/eipd1rm1tilzqy1o815j216mzlx28pmeqt.png)
![k_w=[H^+]^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/wsnbv13ampejswd43auvaozc2tf23ryb3w.png)
![1.0* 10^(-14)=[H^+]^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/dz8s9lz6n54tqu2hrp7ekoefqt1d9sg17g.png)
![[H^+]=1.0* 10^(-7)mol/L](https://img.qammunity.org/2018/formulas/chemistry/high-school/2l2pkndzxcchdsfv0q1zp9hw8l2czmyecm.png)
As we know that, pH is the negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
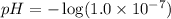
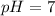
We know that the pH range is, 1 to 14.
When the value of pH is less than 7 then the solution is acidic.
When the value of pH is more than 7 then the solution is basic.
When the value of pH is equal to 7 then the solution is neutral.
Hence, the best statement for the pH of pure water is, It is neutral because the concentration of hydronium ions equals that of hydroxide ions.