Answer: 4 is the subscript in
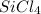 .
.
Step-by-step explanation:
We know that,
Subscript: The number of atom present in a given compound.
In
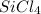 , 4 represents the number of chlorine atom in 1 mole of Silicon tetra chloride.
, 4 represents the number of chlorine atom in 1 mole of Silicon tetra chloride.
Coefficient: Number of atom which is used in reaction.
For example: In
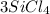 , 3 is the coefficient of silicon tetra chloride.
, 3 is the coefficient of silicon tetra chloride.
A Product : In the balance equation, right hand side is called product.
A reactant : In the balance equation, left hand side is called reactant.
For example: the balance equation is
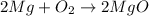
In this equation,
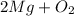 is the reactants and
is the reactants and
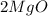 is products.
is products.
Hence, 4 is the subscript in
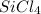 .
.