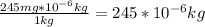Answer:
245*10⁻⁶ kg
Step-by-step explanation:
Given:
Mass of nitrogen in a glass bulb = 245 mg
In order to convert mass from mg (milligrams) to kg (kilograms) use the following relation
1 mg = 0.001 gram = 10⁻³ g
1 g = 0.001 kg = 10⁻³ kg
Therefore,
1 mg = 10⁻⁶ kg
The given mass of 245 mg would correspond to :