Answer:
The correct answer is option A.
Step-by-step explanation:
Pressure of the gas ,P= 0.9 kPa = 0.008882 atm
1 kPa = 0.0098 atm
Number of moles gases = n = 0.04 mol
Temperature of the gas ,T =20°C = 293 K
Volume of the gas = V
Using An Ideal gas equation:
PV=nRT
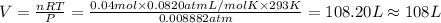
Hence, the correct answer is option A.