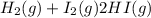Answer: Formation of hydrogen iodide will occur.
Step-by-step explanation:
When molecule of hydrogen gas
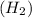 and molecule of iodine gas
and molecule of iodine gas
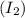 collide with proper orientation and with sufficient amount of energy. This collision of the two different molecules of gases will result in the formation of hydrogen iodide.
collide with proper orientation and with sufficient amount of energy. This collision of the two different molecules of gases will result in the formation of hydrogen iodide.
The balanced chemical equation of the reaction will written as: