Answer:
Number '2' should be placed in the blank before oxygen.
Step-by-step explanation:
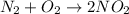
Stoichiometric coefficient is the number mentioned in front of the chemical substances in balanced chemical reaction.
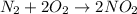
According to stoichiometry, 1 mole of nitrogen gas reacts with 2 moles of oxygen gas to give 2 moles of nitrogen dioxide.
Stoichiometric coefficient of nitrogen gas = 1
Stoichiometric coefficient of oxygen gas = 2
Stoichiometric coefficient of nitrogen dioxide gas = 2