Answer:
Oxidation is defined as the reaction in which there is loss of electrons. It is accompanied by increase in oxidation number.
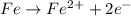
Fe being the pure element in its standard state has oxidation number of zero and on loosing electron changes to oxidation number of +2.
Reduction is defined as the reaction in which there is gain of electrons. It is accompanied by decrease in oxidation number.
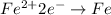
Iron
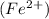 changes from oxidation number of +2 to pure iron [Fe} with oxidation number of zero.
changes from oxidation number of +2 to pure iron [Fe} with oxidation number of zero.