Data:
M (molarity) = ? (M or Mol/L)
m (mass) = 13.50 g
V (volume) = 250 mL → 0.25 L
MM (Molar Mass) of Lead(IV) Nitrate
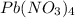
Pb = 1*207 = 207 amu
N = (1*14)*4 = 14*4 = 56 amu
O = (3*16)*4 = 48*4 = 192 amu
------------------------------------
MM of
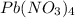
= 207+56+192 = 455 g/mol
Formula:
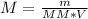
Solving:
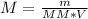
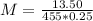
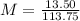
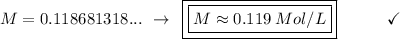
Answer:
B. 0.119 M