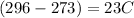Answer:
23C
Step-by-step explanation:
As oxygen is in a gaseous state, and with the data that gives us the problem we must use the equation of ideal gases
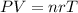
P= pression
V= volumen
n= moles
r= constant of ideal gases
T= temperature
data:
Pression: 123 KPa
Volumen: 10.0L
moles: 0.5 moles
constant of ideal gases:
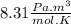
we transform the liters to cubic meters:
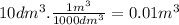
we clear the temperature and replace the data
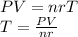
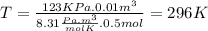
The temperature is 296 K but we have to expressed in Celsius