Answer: Option (C) is the correct answer.
Step-by-step explanation:
Relation between pH and hydrogen ion concentration is as follows.
pH =
![-log[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/56aeusogtgelmztxoavwpz2efojjy6v0ta.png)
It is given that hydrogen ion concentration is
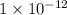 M. Therefore, putting this value in the above formula as follows.
M. Therefore, putting this value in the above formula as follows.
pH =
![-log[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/56aeusogtgelmztxoavwpz2efojjy6v0ta.png)
=
![-log[1 * 10^(-12)]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/czbm2hil6lhmzj6db5gvz4418pkfh31j5h.png)
= - (-12) (log [1] + log[10]) (log[1] = 0 and log[10] = 1)
= 12
As pH = 12, hence the solution will be highly basic. As a result, there will be high concentration of hydroxide ions.