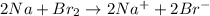Step-by-step explanation:
It is known that when there is gain of electrons then that is reduction reaction. Whereas when there is removal of electrons then that is oxidation reaction.
The given half reactions are as follows.
Reduction half-reaction:
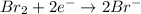 ..... (1)
..... (1)
Oxidation half-reaction:
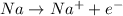 ...... (2)
...... (2)
Balancing the oxidation half reaction by multiplying it by 2 as follows.
Oxidation half-reaction:
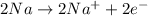 ..... (3)
..... (3)
Therefore, adding equation (1) and (3) by cancelling common terms, we get the balanced redox equation as follows.