Answer:
Iron gets reduced from a +3 oxidation state to 0
Step-by-step explanation:
The decomposition of iron oxide to elemental can be represented by the following equation:

In iron oxide (Fe2O3), the oxidation state of iron is +3 while that of oxygen is -2. Therefore, the above reaction is a redox (reduction oxidation reaction).
Here, iron in iron oxide gains 3 electrons and gets reduced from Fe3+ to Fe(0). Whereas, the oxygen loses 2 electrons and gets reduced from O2- to O(0).
The half reactions can be represented as:
Reduction:
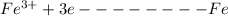
Oxidation:
