Answer : The volume of methyl alcohol is 270.1 mL
Explanation :
As we are given 18.5 % (V/V) methyl alcohol. That means, 18.5 mL volume of methyl alcohol present in 100 mL of solution.
Now we have to determine the volume of methyl alcohol in 1.46 L or 1460 mL solution.
As, 100 mL solution contains 18.5 mL of methyl alcohol
So, 1460 mL solution contains
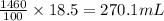 of methyl alcohol
of methyl alcohol
Therefore, the volume of methyl alcohol is 270.1 mL