Answer:
73.0 grams is the mass of two moles of HCl.
Step-by-step explanation:
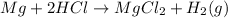
Moles of HCl = 2 moles
Molar mass of HCl = 36.5 g/mol
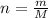
n = number of moles
m = Mass of the given compound
M = molar mass of the compound
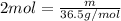
m =
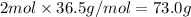
73.0 grams is the mass of two moles of HCl.