Answer:
The final temperature of the gas is 60K
Step-by-step explanation:
The gas law to be used here is Gay-Lussac's law which states that the pressure of a given mass of gas is directly proportional to its temperature in kelvin provided volume remains constant. The law can be represented with the formula below
P₁/T₁ = P₂/T₂
where P₁ is the initial pressure (500 kPa)
T₁ is the initial temperature (300K)
P₂ is the final pressure (100 kPa)
T₂ is the final temperature (unknown)
Hence,
500/300 = 100/T₂
T₂ =
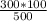
T₂ = 60K
Hence, the final temperature of the gas is 60K