Answer:
50,100Joules
Step-by-step explanation:
The formula for calculating the amount of heat needed to melt to ice is expressed as:
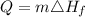
where:
• m is the ,mass of ice, = 150.0g
,
• Heat of ,formation, Hf = 334J/g
Substitute the given parameters into the formula
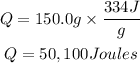
Hence the amount of heat needed to melt a piece of ice is 50,100Joules