Answer: The correct answer is Option A.
Step-by-step explanation:
Covalent compound is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound. These are usually formed when two non-metals react.
An ionic compound is defined as the compound which is formed when electron gets transferred from one atom to another atom. These are usually formed when a metal reacts with a non-metal or a metal reacts with a polyatomic ion or a reaction between two polyatomic ions takes place.
For the given options:
- Option A:
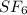
Sulfur and fluorine, both are non-metals and they will form a covalent compound.
- Option B:
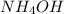
This compound is formed by the combination of
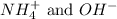 ions. These two are polyatomic ions and thus will form an ionic compound.
ions. These two are polyatomic ions and thus will form an ionic compound.
- Option C:
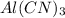
This compound is formed by the combination of aluminium which is a metal and
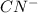 ion which is a polyatomic ion. Thus, it will form an ionic compound.
ion which is a polyatomic ion. Thus, it will form an ionic compound.
- Option D:
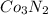
This compound is formed by the combination of cobalt which is a metal and nitrogen which is a non-metal. Thus, it will form an ionic compound.
Hence, the correct answer is Option A.