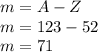Answer:
Helium: 1
Carbon: 2
Nitrogen: 8
Strontium: 52
Tellurium: 71
Step-by-step explanation:
An isotope is atom that belongs to the same chemical element as another, has the same atomic number, but different atomic mass
So the data they give us is the mass number of the isotopes
A= mass number
Z= atomic number ( number of protons in the nucleus).
IS the number that appears in the periodic table
For example, the Z of neon is 10 and the Z of the oxygen is 8
m= number of neutrons
A= Z+ m
m=A-Z
Helium:
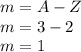
Carbon:
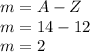
Nitrogen:
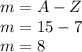
Strontium:
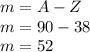
Tellurium: