Answer : The mass of percent of nitrogen in the dyeing agent, picric acid is, 6.11 %
Solution : Given,
Molar mass of nitrogen = 14.01 g/mole
Molar mass of picric acid = 229.11 g/mole
As, we know that in
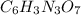 , there are 6 carbon atoms, 3 hydrogen atoms, 3 nitrogen atoms and 7 oxygen atoms.
, there are 6 carbon atoms, 3 hydrogen atoms, 3 nitrogen atoms and 7 oxygen atoms.
Formula used for the mass percent of nitrogen.
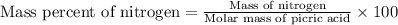
Now put all the given values in this formula, we get the mass of percent of nitrogen.
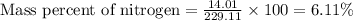
Therefore, the mass of percent of nitrogen in the dyeing agent, picric acid is, 6.11 %