Joseph Priestly is frequently credited with the discovery of oxygen, and was reported to have produced molecular oxygen from the decomposition reaction of mercury(II) oxide, which is the reverse of the synthesis of HgO depicted in the following equation.
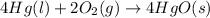 Determine the value of
Determine the value of
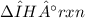 for the synthesis, given that
for the synthesis, given that
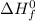 for HgO is -90.7 kJ/mol.
for HgO is -90.7 kJ/mol.
Answer: The enthalpy change for this reaction is, -362.8 kJ
Step-by-step explanation:
The balanced chemical reaction is,
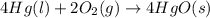
The expression for enthalpy change is,
![\Delta H=\sum [n* \Delta H_f(product)]-\sum [n* \Delta H_f(reactant)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/ki3v3y7u11m1ff8fhba8jdrhiw2jcbq6df.png)
![\Delta H=[(n_(HgO)* \Delta H_(HgO))]-[(n_(O_2)* \Delta H_(O_2))+(n_(Hg)* \Delta H_(Hg))]](https://img.qammunity.org/2022/formulas/chemistry/college/1b3jnlxrucrfjwxieakeb28i521bz6hguq.png)
where,
n = number of moles
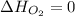 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
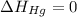 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
Now put all the given values in this expression, we get
![\Delta H=[(4* -90.7)]-[(2* 0)+(4* 0)]](https://img.qammunity.org/2022/formulas/chemistry/college/ttkq6ws56id5j4vwh7ezpcwnemu9yuqgpt.png)
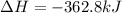
Therefore, the enthalpy change for this reaction is, -362.8 kJ