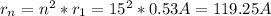Answer:
Step-by-step explanation:
Following the Bohr model radii, where
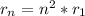 for the hydrogen atom, we can calculate the nth radius if we know the first one, as long as n is a whole number.
for the hydrogen atom, we can calculate the nth radius if we know the first one, as long as n is a whole number.
The problem gives us the first diameter, and we also know that d=2r so
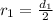
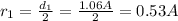
Now we change this value in the Bohr model radii formula: