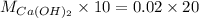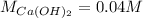Answer: Molarity of Calcium Hydroxide solution is 0.04M.
Explanation: We are given 10.00 mL of Calcium hydroxide solution which is neutralized by 20.00 mL of 0.02M of
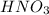 solution.
solution.
To find out the molarity of calcium hydroxide, we use the formula:
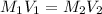
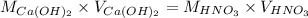
Putting the values in above equation, we get