Answer: The substance formed will be a covalent compound.
Step-by-step explanation:
A covalent compound is defined as the compound which is formed from the sharing of electrons between the element that are combining together.
These compounds are most likely to be formed when two non-metals combine together. For Examples: HCl,
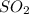 etc.. are covalent compounds.
etc.. are covalent compounds.
Hence, the substance formed will be a covalent compound.