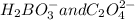Answer : Option D)
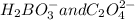 .
.
Explanation : The acid base theory of Bronsted-Lowry, stated the definition of acids and bases on the basis of conjugated.
The fundamental concept of this theory states that when an acid and a base reacts with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchanging the proton.
So, in the given reaction;

It is firstly observed that the species
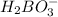 is accepting a proton from the acid, therefore this is one of the bases.
is accepting a proton from the acid, therefore this is one of the bases.
Secondly,
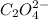 accepts the proton and forms the reactant again in the reversible reaction.
accepts the proton and forms the reactant again in the reversible reaction.
Therefore, these are the correct options.