Answer:
Amount = 2250 g
Step-by-step explanation:
Note: This question is incomplete and lacks each and every data to solve. However, I have found the similar question on the internet and will be solving this question for you. Cheers!
Data Missing:
Organic Compound O
Solubility = 0.225 g/ml
Temperature = 10°C
Calculate the greatest mass of O that can be dissolved in 10.0 L acetonitrile at 10°C temperature.
Solution:
As we know the volume of the acetonitrile = 10.0 L
but first, we need to convert this into mL
We know that,
1 L contains 1000 mL
So,
10 L will contain = 10 x 1000 mL
10 L = 10000 mL
We, can write, 10000 mL as:
1 x
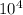 mL
mL
Now, the formula to calculate the greatest amount of O that can be dissolved in 1 x
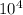 mL acetonitrile at 10°C temperature is:
mL acetonitrile at 10°C temperature is:
Amount = Solubility x volume
Here,
Solubility = 0.225 g/ml
and
Volume = 1 x
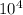 mL
mL
Plugging in the values, we get:
Amount = 0.225 g/ml x 1 x
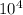 mL
mL
Amount = 0.225 x
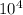 g
g
Amount = 2250 g