Answer: The correct answer is Option b.
Step-by-step explanation:
This solubility of a gas with change in pressure is described by Henry's Law.
This law states that the mass of a gas dissolved in a given volume of the liquid at constant temperature is directly proportional to the pressure of the gas present in equilibrium with the liquid.
Mathematically,
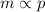
or,
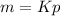
Where, m = mass of the gas dissolved in a unit volume of the solvent
K = Proportionality constant
p = Pressure of the gas in equilibrium with the solvent.
With increase in pressure, the mass of gas dissolved will also increase and vice-versa.
Hence, the correct answer is Option b.