Answer: ammonia
Step-by-step explanation:
Heat capacity is the amount of heat required to raise the temperature of 1 gram of substance through
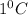 .
.

Q= heat gained= 18.8 kJ
m= mass of the substance = 200 g
c = heat capacity= ?
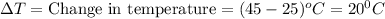
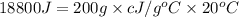

The heat capacity of water is
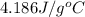 ,ammonia is
,ammonia is
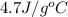 , gasoline is
, gasoline is
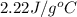 and air is
and air is
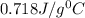 .
.
Thus the correct answer is ammonia.