Answer: Magnesium Chloride
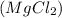 is produced other than hydrogen gas in the following reaction.
is produced other than hydrogen gas in the following reaction.
Step-by-step explanation:
The given reaction is a type of single displacement reaction. This reaction is defined as the reaction in which a more reactive metal displaces a less reactive metal in a chemical reaction.
For the reaction of magnesium and hydrochloric acid, magnesium metal is more reactive than hydrogen, so it will easily displace hydrogen from its chemical reaction.
The equation follows:
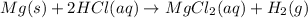
By Stoichiometry,
1 mole of magnesium metal reacts with 2 moles of hydrochloric acid to produce 1 mole of magnesium chloride and hydrogen gas.
Hence, the other product formed in the given reaction is magnesium chloride.