This question uses Ideal Gas Law, whose equation is as follows:
PV = nRT
P = pressure = 1 atm
V = volume = unknown
n = moles = 8.90 mol
R = gas constant =
0.082
T = temperature = 273. K
Since we are solving for Volume, you can rearrange the formula to get V alone:
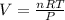
Plug in the given values and solve:

V = 199.2354 L
Round to the necessary significant digits