Answer:
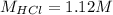
Step-by-step explanation:
Hello!
In this case, for the first reaction we need to focus on, the neutralization of magnesium hydroxide by phosphoric acid, we can write up the following equation:
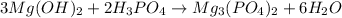
Whereas the acid and base react in a 3:2 mole ratio; thus, we can write:
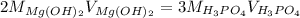
Now, solving for the concentration of the magnesium hydroxide solution we get:
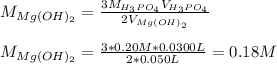
Now, for the reaction between hydrochloric acid and magnesium hydroxide we have:
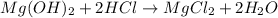
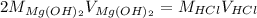
Therefore, solving for the concentration of the hydrochloric acid solution we get:

Best regards!