Answer:
Produce throughout the shorter term but depart the industries run if the circumstances don't start changing because the losses are incurred.
Step-by-step explanation:
The given values are:
Gold sells,
Q = 50
Price,
= $5000
Total cost,
= $300,000
Fixed cost,
= $100,000
So,
⇒
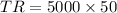
⇒
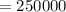 ($)
($)
Now,
⇒
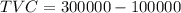
⇒
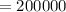
So that,
⇒
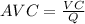
On substituting the values, we get
⇒
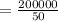
⇒
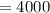
So the above is the correct answer.