Answer: Option (a) is the correct answer.
Step-by-step explanation:
According to Bronsted-Lowry, species which are able to donate or give hydrogen ions are known as acids. Whereas species which accept hydrogen ions are known as bases.
For example,
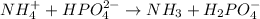
Here
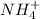 is donating hydrogen ions hence it is the Bronsted-Lowry acid.
is donating hydrogen ions hence it is the Bronsted-Lowry acid.