Answer:
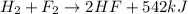
Step-by-step explanation:
Hello!
In this case, since exothermic reactions evolve or release energy when undergone, we infer that the energy is part of the products as it is a result of the reaction; thus, for the described reaction we should set it up as follows:
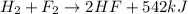
However, if the reaction absorbs energy, the energy associated to the reaction is put at the reactants side, keeping in mind that it would be an endothermic reaction.
Best regards!