Answer : The correct option is, (C) Temperature
Explanation :
Average kinetic energy : Average kinetic energy of the gas particles are directly proportional to the temperature of the gas particles.
Formula used for the average kinetic energy is :
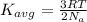
where,
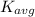 = average kinetic energy
= average kinetic energy
R = gas constant
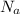 = Avogadro's number or constant
= Avogadro's number or constant
T = temperature of the system
From the formula, we conclude that the average kinetic energy is directly proportional to the temperature. That means temperature defines the average kinetic energy of a systems particles.
Therefore, the correct option is, (C) Temperature