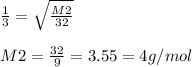Answer:
Molar mass of unknown gas = 4.0 g/mol
Step-by-step explanation:
Based on the Graham's law of diffusion, if R1 and R2 are the diffusion rates for gas 1 and gas 2 with respective molar masses M1 and M2, the rates are related as:
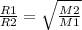 -----(1)
-----(1)
If R1 is the rate of diffusion of O2 and R2 that of the unknown gas, then as per the given information
R2 = 3(R1)
Now, molar mass of O2 = M1 = 32 g/mol. Based on eq(1), substituting for R1, M1 we get: