Answer:
The correct representation of reaction will be:
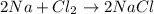
Step-by-step explanation:
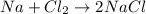
Reaction represented by Andrew is not correct as it is not a chemical reaction balanced. Whenever a chemical reaction takes place mass of the reactants always remains conserved as a per as law of conservation of mass.
The law states that mass can neither be created nor be destroyed. And in a balanced chemical reaction number total mass of reactants is always equal to the total mass of products.
The correct representation of reaction will be:
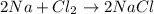
Number of sodium atoms and chlorine atoms on the both sides are equal