So the first thing we must do is write a balanced equation for the reaction and we know the equation is balnced when all the species on the RHS is equal to the species on the LHS
NaOH + HCl → NaCl + H₂O
So now it's time to identify what you know from the question (volume & conc. of NaOH) and use that info to find the unknown (conc. of HCl)
If 1L (1000ml) of NaOH contains 0.50 moles then
let 200ml of NaOH contain x moles
⇒ x =
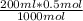
= 0.1 mol
∴ moles of NaOH that reacted with HCl is 0.1 mol
Now mole ratio of NaOH : HCl based on the balance equation is 1 : 1
1NaOH + 1HCl → NaCl + H₂O
∴ if moles of NaOh = 0.1 mol
then moles of HCl = 0.1 mol
<<Alright Now to find the molarity of HCl>>
If 0.1 moles of HCl (which was what took place in the reaction) is in 100 ml
then let x moles would be in 1000ml (1L)
∴ x =
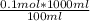
= 1 mol
Thus the concentration of the HCl solution is 1 mol/L
PS. what is in bold is what you really need, the rest is just to help in understanding.