Answer: Heat released during the burning of 0.1625 g of magnesium is 3.79356 kilo Joules.
Step-by-step explanation:
Mass of the magnesium : 0.1625 g
Heat capacity of the bomb calorie-meter,C = 3.03 kJ/°C
Change or increase in temperature
 = 1.252°C
= 1.252°C
Heat =
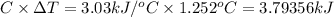
Heat released during the burning of 0.1625 g of magnesium is 3.79356 kilo Joules.