Step-by-step explanation:
As it is given that melting point of copper is
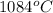 . So, it means liquid state of copper will exist at a temperature greater than
. So, it means liquid state of copper will exist at a temperature greater than
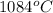 .
.
Whereas, it is known that liquid state of water exists at above zero degree celsius.
And, kinetic energy of the particles of a substance is directly related to the temperature as follows.
K.E =
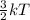
Hence, more is the temperature of a substance more will be the kinetic energy of its molecules.
Therefore, temperature of liquid copper is more than the temperature of liquid water.
Thus, we can conclude that the energy of the particles in a certain amount of liquid copper is greater than the energy of the molecules in the same amount of liquid water.