The energy released when a certain amount of a substance condenses to a liquid is given by:
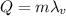
where m is the mass of the substance and
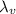 is the latent heat of vaporization.
is the latent heat of vaporization.
In this problem, the mass is m=0.06 kg, while the latent heat of vaporization of mercury is
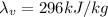 . Therefore, if we apply the formula we get:
. Therefore, if we apply the formula we get:
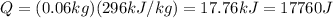
So, the correct answer is
C. 17,705.1 J