Before going to answer this question first we have to understand the basics that lies during the formation of an ionic compound.
Generally an ionic compound is formed between electropositive and electronegative elements.
Normally every element wants to occupy the inert gas configuration in order to be stable.During the ionic bond formation the electropositive metal will lose the electrons and forms cation. The electronegative metal will gain the the electrons and forms anion.
The cation is positively charged and anion is negatively charged. Due to the coulombic force of attraction, the ions will be attracted towards each other and forms ionic compound.
One can take the exampale of NaCl where Na will lose one electron and forms
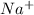 . Chlorine will take one electron and forms
. Chlorine will take one electron and forms
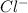 .These ions are attracted and sodium chloride is formed.
.These ions are attracted and sodium chloride is formed.
Hence the formation of an ionic compound follows these steps-
1- First electrons are transferred.
2-The ions are formed
3-The ions are attracted to form the compound
Hence option C is right.