Answer: The smallest bond angle in the molecule x-y-x is
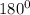
Step-by-step explanation:
According to VSEPR theory , each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom.
In the given compound
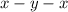 , the number of electron pairs is 2 that means the hybridization will be
, the number of electron pairs is 2 that means the hybridization will be
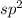 and geometry of the molecule will be linear as there are two bond pairs .The bond angle for linear geometry is 180° which reduces the electron electron repulsions and maximise stability.
and geometry of the molecule will be linear as there are two bond pairs .The bond angle for linear geometry is 180° which reduces the electron electron repulsions and maximise stability.