Answer: The extra energy required is 18.7 J
Step-by-step explanation:
Law of conservation of energy states that energy can neither be created nor be destroyed but it can only be transformed from one form to another form.
We are given:
Energy of Reactant P = 50 J
Energy of Reactant Q = 35.3 J
Energy of Product PQ = 104 J
According to law of conservation of energy:
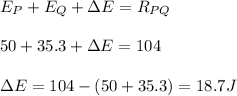
This extra energy is taken up by the reactants.
Hence, the extra energy required is 18.7 J