Answer: a)
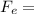 1.99 x 10⁻⁹N
1.99 x 10⁻⁹N
b) Electric force is 1.24 x 10³⁶ times larger than gravitational force
Step-by-step explanation: Electric Force is an interaction between two charges at a distance. It is calculated as
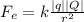
k is electric constant that values 9 x 10⁹N.m²/C²
q and Q are the quantity of charges
r is distance between the charges
a) For the two protons, charge = 1.602 x 10⁻¹⁹C:
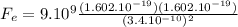
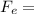 1.99 x 10⁻⁹ N
1.99 x 10⁻⁹ N
Magnitude of electric force between 2 protons is 1.99 x 10⁻⁹ N and it is repulsive because they are both positive
Gravitational Force is the force of attraction that acts between all materials.
It is proportional to the object's masses and inversely proportional to their squared distance:
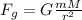
G is gravitational constant that values G = 6.67 x 10⁻¹¹ N.m²/kg²
b) For the two protons, m = 1.67 x 10⁻²⁷kg
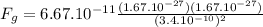
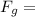 1.61 x 10⁻⁴⁵N
1.61 x 10⁻⁴⁵N
Comparing electrical and gravitational forces:
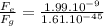
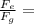 1.24 x 10³⁶
1.24 x 10³⁶
This shows that electrical force is 1.24 x 10³⁶ times greater than gravitational force.