Answer : The correct option is, Ar
Explanation :
(1) The element is,
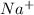
Atomic number of Na = 11
The general electronic configuration of Na is,
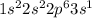
The electronic configuration of
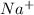 is,
is,
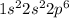
The number of electrons in
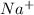 = 10
= 10
(2) The element is,
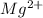
Atomic number of Mg = 12
The general electronic configuration of Mg is,
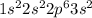
The electronic configuration of
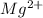 is,
is,
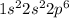
The number of electrons in
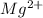 = 10
= 10
(3) The element is,
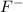
Atomic number of F = 9
The general electronic configuration of F is,
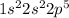
The electronic configuration of
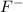 is,
is,
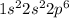
The number of electrons in
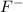 = 10
= 10
(4) The element is, Ar
Atomic number of Ar = 18
The general electronic configuration of Ar is,
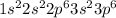
The number of electrons in Ar = 18
(5) The element is,
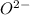
Atomic number of O = 8
The general electronic configuration of O is,
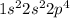
The electronic configuration of
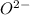 is,
is,
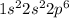
The number of electrons in
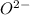 = 10
= 10
From this we conclude that, the option 1, 2, 3 and 5 have the same number of electrons as the neutral atom of neon. While in 'Ar' there are 18 number of electrons.
Hence, the correct option is, Ar