Answer:
D. C2H6
Step-by-step explanation:
1st) It is necessary to calculate the grams of carbon and hydrogen, knowing that the 30.1g represents the 100% of the mass:
• Carbon:
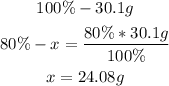
• Hydrogen:
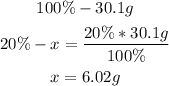
Now we know that there are 24.08g of carbon and 6.02g of hydrogen in the molecule.
2nd) Using the molar mass of carbon (12.04g/mol) and hydrogen (1.003g/mol) we can calculate the moles of each element in the molecule:
• Carbon:
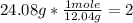
• Hydrogen:
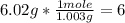
So, the molecular formula is C2H6.